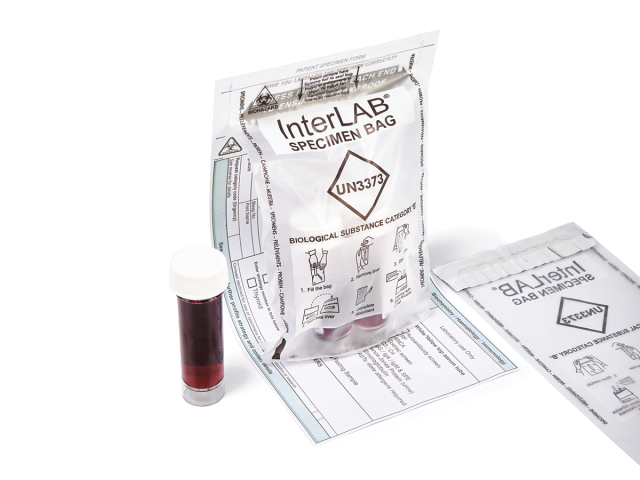
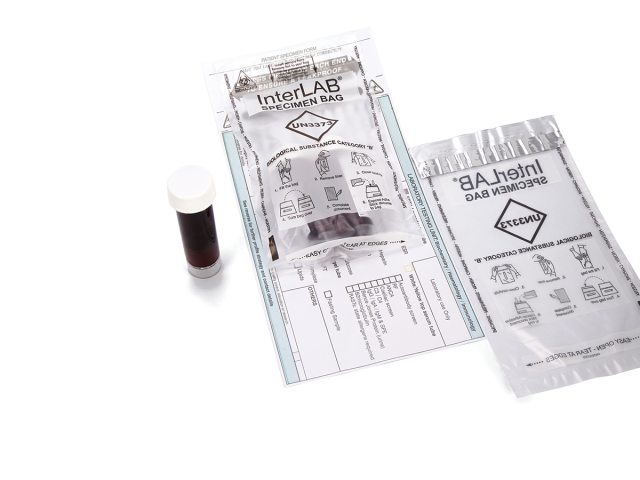
InterLAB® specimen bags are used to provide the safe and secure transportation of samples and category B biological substances, conforming to UN3373 requirements. Compliant with international and European standards for the transport of infectious substances (ADR2017/P650 WHO/HSE/GCR/2012.12 - ISO 15189), InterLAB® bags feature an adhesive glue line with removable release liner to provide a liquid-tight solution to secure specimen samples. Coveris’ InterLAB® bags also feature a peelable adhesive on the reverse to secure vital documentation.
Benefits
- Designed to offer protective and secure transport of samples and specimens
- InterLAB® bags feature a leak resistant closure to avoid risk of cross contamination and protection of nosocomial infections
- Conforms to UN3373 regulations for Biological and Infectious substances
- Recyclable
- Customise with bespoke print and sizing to suit the application
- A secondary packaging solution for a broad range of samples
- Available with a peelable adhesive strip to attach vital requisition documentation for sample identification
- Low carbon footprint alternative to sterile or non-sterile glass, jars and pouches
- Superior replacement to a variety of bags currently in use (e.g. grip, paper & plastic bag combinations) and compatible with ICE systems
Applications
- Animal, human or plant swabs & samples including liquids
- Evidence
- Infectious substances
- Medications
LOCATIONS:
- UK